- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal AJCacjfc. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. 2018;68(6):394-424.
- 2. Memirie ST, Habtemariam MK, Asefa M, et al. Estimates of cancer incidence in Ethiopia in 2015 using population-based registry data. 2018;4:1-11.
- 3. Mauri D, Pavlidis N, Ioannidis JPJJotNCI. Neoadjuvant versus adjuvant systemic treatment in breast cancer: a meta-analysis. 2005;97(3):188-194.
- 4. Bardia A, Baselga JJCCR. Neoadjuvant therapy as a platform for drug development and approval in breast cancer. 2013;19(23):6360-6370.
- 5. Nyblade L, Stockton M, Travasso S, Krishnan SJBwsh. A qualitative exploration of cervical and breast cancer stigma in Karnataka, India. 2017;17(1):1-15.
- 6. Tigeneh W, Molla A, Abreha A, Assefa MJIJCRMM. Pattern of cancer in Tikur Anbessa specialized hospital oncology center in Ethiopia from 1998 to 2010. 2015;1(1):1-5.
- 7. Gajdos C, Tartter PI, Estabrook A, Gistrak MA, Jaffer S, Bleiweiss IJJJoso. Relationship of clinical and pathologic response to neoadjuvant chemotherapy and outcome of locally advanced breast cancer. 2002;80(1):4-11.
- 8. Shohdy KS, Almeldin DS, Fekry MA, et al. Pathological responses and survival outcomes in patients with locally advanced breast cancer after neoadjuvant chemotherapy: a single-institute experience. 2021;33(1):1-10.
- 9. Alawad AAMJEjohs. Evaluation of clinical and pathological response after two cycles of neoadjuvant chemotherapy on sudanese patients with locally advanced breast cancer. 2014;24(1):15-20.
- 10. Boughey JC, McCall LM, Ballman KV, et al. Tumor biology correlates with rates of breast-conserving surgery and pathologic complete response after neoadjuvant chemotherapy for breast cancer: findings from the ACOSOG Z1071 (Alliance) Prospective Multicenter Clinical Trial. 2014;260(4):608.
- 11. Woo J, Oh SJ, Song J-Y, et al. Response to neoadjuvant chemotherapy based on pathologic complete response in very young patients with ER-positive breast cancer: a large, multicenter, observational study. 2021;21(1):1-11.
- 12. Golshan M, Cirrincione CT, Sikov WM, et al. Impact of neoadjuvant therapy on eligibility for and frequency of breast conservation in stage II–III HER2-positive breast cancer: surgical results of CALGB 40601 (Alliance). 2016;160(2):297-304.
- 13. Van J, Hage C, Velde J, et al. Preoperative chemotherapy in primary operable breast cancer: results from the European Organization for Research and Treatment of Cancer trial 10902. 2001;33.
- 14. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. 2014;384(9938):164-172.
- 15. Spring LM, Fell G, Arfe A, et al. Pathologic complete response after neoadjuvant chemotherapy and impact on breast cancer recurrence and survival: a comprehensive meta-analysis. 2020;26(12):2838-2848.
- 16. Kinsella MD, Nassar A, Siddiqui MT, Cohen CJIjoc, pathology e. Estrogen receptor (ER), progesterone receptor (PR), and HER2 expression pre-and post-neoadjuvant chemotherapy in primary breast carcinoma: a single institutional experience. 2012;5(6):530.
- 17. Amin M, Edge S, Greene F. et alAJCC Cancer Staging Manual: New York: Springer; 2017.
- 18. Mischel A-M, Rosielle DAJJoPM. Eastern Cooperative Oncology Group Performance Status# 434. 2022;25(3):508-510.
- 19. Nishino M, Jagannathan JP, Ramaiya NH, Van den Abbeele ADJAJoR. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. 2010;195(2):281-289.
- 20. Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. 2000;92(3):205-216.
- 21. Sasanpour P, Sandoughdaran S, Mosavi-Jarrahi A, Malekzadeh MJAPJoCPA. Predictors of pathological complete response to neoadjuvant chemotherapy in Iranian breast cancer patients. 2018;19(9):2423.
- 22. Simos D, Clemons M, Ginsburg OM, Jacobs CJCOiS, Care P. Definition and consequences of locally advanced breast cancer. 2014;8(1):33-38.
- 23. Lee MC, Newman LAJSCoNA. Management of patients with locally advanced breast cancer. 2007;87(2):379-398.
- 24. Rahman MS, Akhter PS, Hasanuzzaman M, et al. Outcome of neoadjuvant chemotherapy in locally advanced breast cancer: A tertiary care center experience. Bangladesh Med J. 2017;45(3):141–146.
- 25. Bhattacharyya T, Sharma SC, Yadav BS, Singh R, Singh G. Outcome of neoadjuvant chemotherapy in locally advanced breast cancer: A tertiary care centre experience. Indian J Med Paediatr Oncol. 2014 Jul;35(3):215-20.
- 26. Schlichting JA, Soliman AS, Schairer C, et al. Breast cancer by age at diagnosis in the Gharbiah, Egypt, population-based registry compared to the United States Surveillance, Epidemiology, and End Results Program, 2004-2008. Biomed Res Int. 2015;2015:381574.
- 27. Redkar AA, Kabre SS, Mitra I. Estrogen and progesterone receptors measurement in breast cancer with enzyme-immunoassay and correlation with other prognostic factors. Indian J Med Res. 1992;96:1-8.
- 28. Alvarado-Cabrero I, Alderete-Vazquez G, Quintal-Ramirez M, Patino M, Ruiz E. Incidence of pathologic complete response in women treated with preoperative chemotherapy for locally advanced breast cancer: correlation of histology, hormone receptor status, Her2/Neu, and gross pathologic findings. Ann Diagn Pathol. 2009;13(3):151-7.
- 29. Asselain B, Barlow W, Bartlett J, Bergh J, Bergsten-Nordström E, Bliss J, et al. Long-term outcomes for neoadjuvant versus adjuvant chemotherapy in early breast cancer: meta-analysis of individual patient data from ten randomised trials. Lancet Oncol. 2018 Jan;19(1):27–39.
- 30. Abdel-Bary N, El-Kased AF, Aiad HAZ. Does neoadjuvant chemotherapy increase breast conservation in operable breast cancer: an Egyptian experience. E Cancer Med Sci. 2009;3:104.
- 31. El-Sayed MI, Maximous DW, Zakhary MM, Mikhail NNH. Biological markers and response to neoadjuvant taxane-based chemotherapy in patients with locally advanced breast cancer. ISRN Oncol. 2012;2012:245891.
- 32. Resende U, Cabello C, Oliveira Botelho Ramalho S, Zeferino LC. Predictors of Pathological Complete Response in Women with Clinical Complete Response to Neoadjuvant Chemotherapy in Breast Carcinoma. Oncology. 2018;95(4):229-238. doi: 10.1159/000489785. Epub 2018 Jul 19. PMID: 30025385.
Journal Menu
Articles
Useful links
- Peer Review
- Why Submit?
- Submission Checklist
- Article Types
- Instructions for Authors
- Article Processing Fee
Home Menu
Assessment of Pathological Complete Response and Clinical Responses in Locally Advanced Breast Cancer after Neoadjuvant Chemotherapy
Affiliations
Addisababa University, Department of Clinical Oncology, Ethopia.
*Corresponding Author: Miliyard Demeke, Addisababa University,Department of Clinical Oncology, Ethopia.
Citation: Demeke M. Assessment of Pathological Complete Response and Clinical Responses in Locally Advanced Breast Cancer after Neoadjuvant Chemotherapy.Collect J Oncol. Vol 2 (1) 2025; ART0085.
Abstract
Background: The use of neoadjuvant chemotherapy in treating breast cancer has shown efficacy in down staging primary tumors and allows breast conservative surgery to be performed instead of mastectomy. This study aims to evaluate clinical and pathological response after the use of neoadjuvant chemotherapy in patients with locally advanced breast cancer.
Methods: This is a cross-sectional study of forty-one patients who presented from January 1st, 2021 through June 2022 with locally advanced breast cancer and treated with neoadjuvant chemotherapy were included.
Results: In our study included 41 patients with a median age of 41 years. The cumulative clinical response rate was 75%; nine patients (22%) had a complete clinical remission (cCR); 22 had a partial remission (53.3%); six had stable disease (14.6%), and four had progressive disease (9.8%). Seven patients (18.9%) had complete pathological response.
Conclusions: Neoadjuvant chemotherapy resulted in high clinical response with complete pathological response in some patients with locally advanced breast cancer. We recommend further research to find predictors for response.
Acronyms/Abbreviations
AA: Addis Ababa, AACCR: Addis Ababa City Cancer Registry, AAU: Addis Ababa University, AJCC: American Joint Committee on Cancer, ASC: Adenosquamous Carcinoma, cCR: Complete clinical remission, CHS: College of Health Science, CI: Confidence Interval, CR: Complete response, CXR: Chest X-Ray, CT: Computed Tomography / Chemotherapy, DM: Distant metastases, EFS: Event free survival, ECOG: Eastern Cooperative Oncology Group, GLOBOCAN: Global Burden of Cancer Study, LABC: Locally advanced breast cancer, NAC: Neoadjuvant chemotherapy, OS: Overall survival, pCR: Pathological complete response, TASH: Tikur Anbessa Specialized Hospital, TNM: Tumor, Nodes, Metastasis
Introduction
Cancer is one of the leading causes of death worldwide. It is the second most common cause of death globally, accounting for an estimated 9.6 million deaths in 2018[1]. According to GLOBOCAN 2018, there were an estimated 18.1 million new cancer cases (excluding 17.0 million non-melanoma skin cancers) and 9.6 million cancer deaths (excluding 9.5 million non-melanoma skin cancers) worldwide[1]. Globally, breast cancer is the most frequently diagnosed cancer and the leading cause of cancer death in women, with approximately 2 million new cases and nearly 626,000 related deaths in 2018[1]. In Ethiopia, breast cancer is by far the most common cancer. The first data from the Addis Ababa City Population-Based Cancer Registry shows that breast cancer is the most common cancer in women and one of the top ten cancers in men, constituting 33% of cancers in women and 23% of all cancers identified in the registry[2]. Neoadjuvant chemotherapy (NAC) has traditionally been used to downstage locally advanced breast cancer to make it operable, but it is now also used in managing localized breast cancer as an alternative to adjuvant chemotherapy. Studies have shown that the benefit of chemotherapy is similar when given in the adjuvant or neoadjuvant setting, with no difference in overall survival[3]. However, NAC offers several additional advantages from both clinical and research perspectives. In patients with large tumors, NAC has the potential to reduce tumor size and improve the rate of breast conservation surgery (BCS), while also leading to less extensive axillary surgery[4]. Because the primary tumor remains intact during therapy, the neoadjuvant approach allows for monitoring of treatment response and gathering information on in-vivo chemosensitivity, including the possibility to switch therapy if the response is inadequate[4].
Statement of the Problem
Locally advanced breast cancer (LABC) is a very common clinical scenario, especially in developing countries. This is likely due to various factors such as lack of education, limited public awareness about cancer, absence of community screening programs, personal and social stigma, societal taboos surrounding cancer, and poor socioeconomic status[5]. In Ethiopia, most patients with cancer—including breast cancer—present at advanced stages. LABC constitutes 67% of new breast cancer cases in the country[6].
Justification of the Study
The incidence of breast cancer in Ethiopia is increasing, with the majority of patients presenting at advanced stages when upfront surgery is not usually an option. Consequently, patients with LABC are commonly initiated on neoadjuvant chemotherapy (NAC) followed by surgery. Identifying subsets of patients who are most likely to benefit from NAC requires understanding predictors of pathological complete response (pCR) and clinical responses. Despite the well-described influence of racial disparities on NAC outcomes, there is limited data characterizing these predictors among Ethiopian breast cancer patients. The aim of this study was to assess pCR and clinical responses, and identify associated factors among breast cancer patients receiving NAC at our institution. To our knowledge, there are no published studies from Ethiopia identifying predictors of pCR or clinical response in this patient population.
Literature Review
Pathological complete response and clinical response are considered surrogate markers for NAC efficacy in LABC. Csaba (2002) retrospectively studied 144 LABC patients treated with NAC and found complete clinical response in 8% and pCR in 13%. Smaller tumors responded more favorably. Distant disease-free survival (P = 0.039) and overall survival (P = 0.035) were significantly associated with the number of involved axillary lymph nodes[7]. A study from Egypt analyzed predictive clinical factors for pathological response and survival. Among patients with clinical T3–T4 tumors (58%) and 80% with positive axillary nodes, the objective response rate (ORR) was 78%, and pCR was achieved in 16%. Higher ORR was associated with clinical node stage (P = 0.035) and receiving optimal chemotherapy (P = 0.001). Predictors of pCR included clinical T-stage (P = 0.026), high Ki-67 index >20 (P = 0.05), and optimal chemotherapy (P = 0.014). Achieving pCR was linked to better disease-free survival (DFS), with hazard ratios of 0.56 (P = 0.008), 0.38 (P = 0.04), and 0.007, respectively[8]. A prospective study from Sudan evaluated clinical and pathological response after two NAC cycles in LABC patients. Of 98 patients, clinical response was 83%: 11.2% had complete clinical remission (cCR), 72.4% partial response, 13.3% stable disease, and 3.1% progressive disease. Seven achieved pCR[9]. Triple-negative and HER2-positive breast cancers show the highest rates of breast-conserving surgery and pCR post-NAC. The ACOSOG Z1071 trial (n=756) found breast-conserving surgery rates of 46.8% in triple-negative and 43.0% in HER2-positive cases, compared to 34.5% in hormone receptor-positive/HER2-negative tumors (P = 0.019). pCR rates were 38.2% (triple-negative), 45.4% (HER2-positive), and 11.4% (hormone receptor-positive/HER2-negative) (P < 0.0001)[10]. Joohyun et al. conducted a multicenter study on 1,048 ER-positive and 797 ER-negative patients under 50. Breast conservation rates were similar across ages. However, pCR rates were significantly higher in younger (<35 years) ER-negative patients (P = 0.009), but not in ER-positive patients (P = 0.71), suggesting better NAC response in younger ER-negative patients[11]. The CALGB 40601 (Alliance) trial demonstrated NAC's role in increasing eligibility for breast-conserving therapy (BCT). Among 292 patients, 59% were non-BCT candidates at baseline. NAC converted 43% to BCT eligibility, and 67% of them opted for BCT with an 80% success rate. Common pre-NAC ineligibility factors such as tumor size (56%) and anticipated poor cosmetic outcomes (2(26%) were reduced by 67 and 75%, respectively, with treatment, while multicentricity, the second most common factor (33%), fell by only 16%. Since [12] the use of neoadjuvant chemotherapy early breast cancer yields similar results in terms of PFS, OS, and locoregional control compared with conventional postoperative chemotherapy.In addition to improving tumor response, NAC enables more patients to undergo breast-conserving surgery. The EORTC Trial 10902 enrolled 698 breast cancer patients (T1c–T4b, N0–N1, M0) and evaluated the effect of NAC on long-term outcomes. At a median follow-up of 56 months, there were no significant differences between the neoadjuvant and adjuvant arms in terms of overall survival (OS) (HR = 1.16; P = .38), progression-free survival (PFS) (HR = 1.15; P = .27), and time to locoregional recurrence (LRR) (HR = 1.13; P = .61). Notably, 23% of patients were downstaged with preoperative chemotherapy, but 18% still required mastectomy instead of the initially planned breast-conserving therapy[13]. Pathological complete response (pCR) is increasingly recognized as a surrogate endpoint for improved long-term outcomes. The CTNeoBC pooled analysis compiled data from 12 international trials involving 11,955 patients. Tumor eradication from both the breast and lymph nodes was more strongly associated with improved event-free survival (EFS) (HR = 0.44; 95% CI, 0.39–0.51) and OS (HR = 0.36; 95% CI, 0.30–0.44) than eradication from the breast alone (EFS: HR = 0.60; 95% CI, 0.55–0.66; OS: HR = 0.51; 95% CI, 0.45–0.58). The strongest association between pCR and long-term outcomes was observed in patients with triple-negative breast cancer (EFS: HR = 0.24; OS: HR = 0.16) and HER2-positive, hormone receptor-negative tumors treated with trastuzumab (EFS: HR = 0.15; OS: HR = 0.08)[14]. A comprehensive meta-analysis including 27,895 patients further confirmed that achieving pCR following neoadjuvant therapy (NAT) is associated with significantly better EFS (HR = 0.31; 95% PI, 0.24–0.39) and OS (HR = 0.22; 95% PI, 0.15–0.30). This benefit was especially notable among patients with triple-negative (HR = 0.18; 95% PI, 0.10–0.31) and HER2-positive (HR = 0.32; 95% PI, 0.21–0.47) subtypes. Interestingly, the improved EFS associated with pCR was consistent regardless of whether patients received additional adjuvant chemotherapy (HR = 0.36 in both groups), with no statistically significant difference between them (P = 0.60)[15]. Hormone receptor (HR) status, including estrogen receptor (ER) and HER2, is typically stable before and after NAC. A single-institutional experience examined 38 breast carcinoma cases and found ER status unchanged in 45% of tumors before and after NAC (P = 1.00). However, progesterone receptor (PR) positivity decreased significantly from 37% to 21% post-treatment (P = 0.03). For HER2, 32% were positive pre-treatment and 22% post-treatment by IHC (P = 0.20), and for those tested by FISH, positivity declined from 71% to 57% (P = 0.32)[16].
General Objective
To describe magnitude of Pathological complete response and clinical responses in locally advanced breast cancer patients receiving NAC.
- Specific objectives:To describe Pathological complete response in locally advanced breast cancer patients receiving NAC.
- To describe clinical response in LABC.
- To describe factors associated with PCR in LABC.
- To describe factors associated with clinical response in LABC.
Methodology
Study Design
Institution-based cross-sectional study design was used.
Study Area and Period
The study was conducted at the Oncology Department of Tikur Anbessa Specialized Hospital from January 1st, 2021 to June 1st, 2022.Tikur Anbessa Specialized Hospital (TASH) is a tertiary hospital located in Addis Ababa, the capital city of Ethiopia. It is the largest and oldest public hospital in the country, providing high-level clinical care for millions of people and serving as a training ground for health science students from Ethiopia and the Horn of Africa. The hospital hosts the country’s leading oncology center, which delivers specialist care to an estimated catchment population of approximately 100 million. It offers both palliative and curative treatments for patients with histopathologically confirmed malignancies, including breast cancer. The Clinical Oncology Department of TASH is among the most frequently visited units within the hospital. On average, the department evaluates at least 10,000 cancer patients annually. It provides oncologic services to a broad population characterized by diverse demographic, clinical, and social backgrounds.
Study Population
Patients diagnosed locally advanced breast cancer and took NACT at Tikur Anbessa specialized Hospital oncology center from January 1st, 2021 to June 1, 2022.
Inclusion and Exclusion Criteria
Inclusion criteria
- Biopsy confirmed locally advanced breast cancer cases
- Patients who are on treatment in Tikur Anbessa Hospital oncology center
- Adequate clinical, laboratory and imaging information
Exclusion criteria
- Patients outside the study period
- Patients who refused to be part of the study
- Patient discontinued NACT
Study Variables
Dependent variable
- Clinical response
- Pathological response
Independent variables
- Demographic characteristics such as sex, and age
- Clinical profile of breast cancer patients
- Tumor characteristics and stage of the disease
Sampling Methods
All eligible patients with diagnosis of locally advanced breast cancers will be included into the study.
Sample Size Determination
All patients who took NACT for locally advanced breast cancer from January 1st, 2021 to June 1 2022 and fulfilled the inclusion criteria were considered in the study without the need to do a separate sample size calculation
Data Collection Tools and Procedures
Data was collected using a structured checklist with closed-ended questions designed specifically for the study. The tool was adapted from literature review of similar studies. Data was extracted from medical charts by two trained health professionals under the close supervision of the principal investigator. Daily quality checks were conducted to ensure accuracy and completeness.
Operational Definitions
Staging: Breast cancer stage classification based on the AJCC TNM staging manual (8th edition, 2017). It was determined clinically through imaging for pre-operative cases, and pathologically for post-operative cases. Performance Status: Functional assessment using standard scales to determine treatment eligibility and predict prognosis. Treatment Response: Evaluation of cancer response to treatment using clinical signs, imaging, and tumor markers. The RECIST criteria is widely used for assessing treatment response in solid tumors. Complete Pathological Response (pCR): Defined as the absence of residual invasive tumor in the breast surgical specimen post-neoadjuvant therapy. Cases with only ductal carcinoma in situ (DCIS) are also considered to have achieved pCR[21]. Locally Advanced Breast Cancer (LABC): Includes patients with Stage IIB (T3N0) and Stage IIIA to IIIC disease. This comprises T3 (>5 cm) or T4 tumors with chest wall fixation or skin involvement...ulceration and N2/N3 disease (matted axillary and/or internal mammary metastases)[22,23].
Data Quality Assurance
An English version of the checklist was used for data collection. Brief training was provided to two health professionals who served as data collectors. Continuous supervision was maintained during data collection, and the completed checklists were double-checked daily by the data collectors and the principal investigator for consistency and completeness.
Methods of Data Analysis
Data were analyzed using SPSS Statistics software version 26 (SPSS Inc., Chicago, IL). Descriptive statistics including frequency, mean, median, percentiles, and quartile ranks were applied. The chi-square test was used to test associations, with the level of significance set at 5%.
Ethical Considerations
Ethical approval was obtained from the ethical review board of the Clinical Oncology Department. Verbal consent was secured before conducting phone interviews. Patient confidentiality was maintained throughout the study. The researcher and data collectors adhered to ethical guidelines, ensuring participants were protected from any potential harm. No patient names were recorded, and data collection strictly followed the study objectives.
Chapter Five: Result
Socio-Demographic Characteristics
A total of 41 patients were included in the study. Among them, 40 (97.6%) were female and only one (2.4%) was male. Participants' ages ranged from 22 to 71 years, with a mean age of 41.93 ± 11.18 years. Regarding residence, 23 (56.1%) were from Addis Ababa, while 18 (43.9%) came from outside the city. In terms of marital status, 27 (65.9%) were married, 7 (17.1%) were divorced or widowed, and 7 (17.1%) were single. As for occupation, 11 (26.8%) were civil servants, 6 (14.6%) daily laborers, 3 (7.3%) farmers, 2 (4.9%) had other jobs, and 19 (46.3%) were housewives. (See Table 1)
Clinical Characteristics of Patients and Workup at Diagnosis
At presentation, all study participants reported a breast lump as their primary complaint. On physical examination, 27 (65.9%) were found to have a breast lump only, while 14 (34.1%) had both a breast lump and axillary lymphadenopathy.
Table 1: Socio demographic data
| Variable | Frequency(n) | Percent (%) |
|---|---|---|
| Age Min=22, max=71, mean ± SD 41.93±11.18 Median = 41 | ||
| <50 yr | 31 | 75.6% |
| >=50 yr | 10 | 24.4% |
| Gender | ||
| Female | 40 | 97.6% |
| Male | 1 | 2.4% |
| Job | ||
| Civil servant | 11 | 26.8% |
| Daily laborer | 6 | 14.6% |
| Farmer | 3 | 7.3% |
| Merchant | 2 | 4.9% |
| House wife | 19 | 46.3% |
| Marital status | ||
| Married | 27 | 65.9% |
| Divorced/widowed | 7 | 17.1% |
| Single | 7 | 17.1% |
| Address | ||
| A. A | 23 | 56.1% |
| Outside A. A | 18 | 43.9% |
| Religion | ||
| Orthodox | 25 | 61% |
| Protestant | 6 | 14.6% |
| Muslim | 10 | 24.4% |
Table 2: Clinical Characteristics of Patients and Workup at Diagnosis
| Variable | Frequency (n) | Percent (%) |
|---|---|---|
| Comorbidity | 8 | 19.5% |
| No | 33 | 80.5% |
| Complaint at presentation | Breast lump | 100% |
| Pertinent P/E finding | Breast lump | 65.9% |
| Breast lump + axillary LAP | 14 | 34.1% |
| ECOG at presentation | 0 | 9.8% |
| I | 36 | 87.8% |
| II | 1 | 2.4% |
| Clinical TNM stage | ||
| cT stage | ||
| T2 | 1 | 2.4% |
| T3 | 5 | 12.2% |
| T4 | 35 | 85.4% |
| cN stage | ||
| N0 | 16 | 39.0% |
| N1 | 21 | 51.2% |
| N2 | 4 | 9.8% |
| Clinical group stage | ||
| Stage IIb | 2 | 4.9% |
| Stage IIIa | 8 | 19.5% |
| Stage IIIb | 31 | 75.6% |
| Diagnostic work up | ||
| Breast imaging, n=8 | ||
| Mammography | 3 | 7.3% |
| Breast U/S | 5 | 12.2% |
| FNAC | ||
| Ductal carcinoma | 38 | 92.7% |
| Lobular carcinoma | 1 | 2.4% |
| Malignant Breast carcinoma | 2 | 4.9% |
| Core needle biopsy | ||
| Invasive Ductal carcinoma | 3 | 7.3% |
| Not done | 38 | 92.7% |
Additionally, 8 (19.5%) had comorbidity and almost all 40 (97.6%) patients had ECOG 0-1 while only one (2.4%) patient had ECOG 2 at presentation. Clinically, cT4 disease represented 85.4% (n=35), cT3 12.2% (n=5), and axillary node-positive were 61% (n=25) from which 21 (51.2%) and 4 (9.8%) were cN1 and cN2 respectively, at presentation. Considering clinical stage of the disease at presentation, 31 (75.6%) had stage IIIb disease, 8 (19.5%) had stage IIIa disease and the rest 2 (4.9%) had stage IIb disease. (See Table 2).
Among the study participants, all had FNAC biopsy, and 3 (7.3%) had both FNAC and Core Needle Biopsy (CNB). Almost all patients 38 (92.7%) FNAC results were reported as ductal carcinoma, and only 1 (2.4%) was lobular carcinoma and the rest 2 (4.9%) were reported as “malignant breast carcinoma”. All CNB results were reported as invasive ductal carcinoma (IDC) (See Table 2).
Treatment Regimens, Surgical Interventions and Clinical Response
Regarding the treatment, majority of treatment decisions 85.4% were made on MDT (multidisciplinary meeting), while 12.2% and 2.4% were individually decided by oncologist and surgeon respectively. Considering the type of treatment regimens, ACT-T was given for 37 (90.2%) patients, AC was given for 3 (7.3%) and FAC was given only in one case.
Among the study participants, majority 32 (78%) of patients received eight cycles of NACT, while eight (14.6%) and one (2.4%) received four cycles and seven cycles respectively. Whereas, regarding surgical intervention following chemotherapy, MRM was done in 37 (90.2%) of cases and in the rest 4 (9.8%) cases were not operated (Table 3).
On mid-cycle partial clinical response was seen in 30 (73.2%) cases, 10 (24.4%) were stable, and complete response was seen only in one (2.4%) case. In majority of cases 33 (80.5%) the mid-cycle treatment plan was to continue the NACT treatment whereas, in 8 (19.5%) cases definitive surgery was planned. All patients had ECOG-1 on mid-cycle assessment (Table 3).
At end-cycle clinical assessments of patients, partial clinical response accounted 22 (53.7%), complete clinical response accounted for 9 (22%), local progression of tumor seen in 4 (9.8%) and 6 (14.6%) cases had stable disease. All patients had ECOG-1 on end-cycle assessment. Overall,
Table 3: Treatment decisions
| Variable | Frequency (n) | Percent (%) |
|---|---|---|
| First treatment decision after diagnosis | ||
| MDT | 35 | 85.4% |
| Oncologists | 5 | 12.2% |
| Surgeon | 1 | 2.4% |
| Type of Surgery | ||
| MRM | 37 | 90.2% |
| Inoperable | 4 | 9.8% |
| Type of CT given | ||
| ACT-T | 37 | 90.2% |
| AC | 3 | 7.3% |
| FAC | 1 | 2.4% |
| Cycles of CT given | ||
| 8 cycles | 32 | 78.0% |
| 4 cycles | 8 | 19.5% |
| 7 cycles | 1 | 2.4% |
| Complete | 1 | 2.4% |
| Mid cycle clinical response | ||
| Partial | 30 | 73.2% |
| Stable | 10 | 24.4% |
| ECOG at midcycle | Stage I | 41 (100%) |
| Mid cycle plan | ||
| Continue same Rx | 33 | 80.5% |
| Surgery | 8 | 19.5% |
| ECOG at end cycle | Stage I | 41 (100%) |
| End cycle response | ||
| Complete | 9 | 22.0% |
| Partial | 22 | 53.7% |
| Progression (local) | 4 | 9.8% |
| Stable | 6 | 14.6% |
| Clinical response | ||
| Yes (Partial & complete) | 31 | 75.6% |
| No (Stable & progression) | 10 | 24.4% |
| End cycle plan | ||
| Surgery | 37 | 90.2% |
| Second line CT | 3 | 7.3% |
| Hormonal therapy | 1 | 2.4% |
| Sequence of treatment given | ||
| NACT followed by Surgery | 29 | 70.7% |
| NACT followed by Surgery followed by CT | 8 | 19.5% |
| NACT → second line CT or HRT (progressed cases) | 4 | 9.8% |
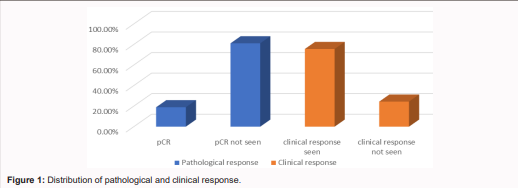
Patterns of the sequences of treatment received by the patients were: 29 (70.7%) NACT followed by surgery, 8 (19.5%) NACT followed by surgery followed by chemotherapy, and NACT followed by second line or hormonal therapy (HRT) accounted 9.8% (4) given for patients having progressed or stable disease. Finally, in majority of cases 37 (90.2%) the end-cycle plan was surgery, while second line CT was planned in 3 (7.3%) cases, and HRT was planned in only one case (Table 3 and Figure 1).
Pathologic Response and Pathologic Parameters
Infiltrative ductal carcinomas accounted for 26 (86.7%) of the cases, while 3 (10%) had infiltrative lobular and only 1 (3.3%) was mucinous carcinoma. From the total study participants, for 11 (26.8%) the histologic subtype of the original tumor was unknown, either because it was originally diagnosed by FNAC, complete pathologic response was seen at the time of definitive surgery, or surgery was not done due to progression at end-cycle response assessment. Among patients with known histologic tumor types, pathologic grade was assessed: 12 (40%) were poorly differentiated (grade 3), 10 (33.3%) were well differentiated (grade 1), and 8 (26.7%) were moderately differentiated (grade 2). Margin status was reported as free (not involved) in 23 (76.7%) cases and positive (involved) in 4 (13.3%) cases, while in 3 (10%) of cases the margin status was not stated in the pathology report. Lymphovascular invasion (LVSI) and perineural invasion (PNI) were reported in 56.7% (17) and 53.4% (16) of cases, respectively. Specifically, LVSI was seen in 11 (36.7%) and PNI in 8 (26.7%) of cases (Table 4). Regarding pathologic staging, pT2 was found in 9 (30%), pT3 in 10 (33.3%), and pT4 in 11 (36.7%) cases. Pathologic nodal status showed pN0 in 3 (10%), pN1 in 19 (63.3%), and pN2 in 8 (26.7%) of cases. However, the number of lymph nodes harvested was inadequate in the majority of cases.
Table 4: Pathological parameter and pathologic stage
| Variable | Frequency, n | Percent, % |
|---|---|---|
| Pathological response, n=37 | ||
| Complete response seen | 7 | 18.9% |
| Complete response not seen | 30 | 81.1% |
| Histologic sub-type, n=30 | ||
| IDC | 26 | 86.7% |
| ILC | 3 | 10.0% |
| Mucinous | 1 | 3.3% |
| Grade(differentiation) | ||
| Well | 10 | 33.3% |
| Moderately | 8 | 26.7% |
| Poorly | 12 | 40.0% |
| Margin status | ||
| Not involved | 23 | 76.7% |
| Involved | 4 | 13.3% |
| Not reported | 3 | 10.0% |
| Hormonal status | ||
| ER PR+ve Her2-ve | 5 | 16.7% |
| Her2+ve | 2 | 6.6% |
| Triple –ve | - | - |
| Not done | 23 | 76.7% |
| Number of LN harvested | ||
| Adequate | 13 | 43.3% |
| Inadequate | 17 | 56.7% |
| LVSI | ||
| Yes | 11 | 36.7% |
| No | 6 | 20.0% |
| Not reported | 13 | 43.3% |
| PNI | ||
| Yes | 8 | 26.7% |
| No | 8 | 26.7% |
| Not reported | 14 | 46.7% |
| Pathologic staging | ||
| T | ||
| T2 | 9 | 30.0% |
| T3 | 10 | 33.3% |
| T4 | 11 | 36.7% |
| N | ||
| N0 | 3 | 10.0% |
| N1 | 19 | 63.3% |
| N2 | 8 | 26.7% |
| Pathologic stage group | ||
| Stage IIA | 1 | 3.3% |
| Stage IIB | 9 | 30.0% |
| Stage IIIB | 8 | 26.7% |
| Stage IIIC | 12 | 40.0% |
In the majority of cases, 17 (56.7%) had inadequate lymph node harvest. Considering the pathologic stage group, 40% (12) of cases were stage IIIc, 26.7% (8) were stage IIIb, 30% (9) were stage IIb, and one case was stage IIa. Hormonal status was assessed only in 7 (23.3%) of cases, among which 5 (16.7%) were ER, PR (+ve), HER2 (-ve), and 2 (6.6%) were HER2 (+ve) (Table 4). Overall, among patients who had MRM, the pathologic response rates were as follows: complete pathologic response was observed in 18.9% (7), while non-complete pathologic response was seen in 81.1% (30) (Table 4, Figure 1).
Determining Factors of Clinical and Pathologic Response
Clinical response (complete and partial) was significantly related to cN stage at the time of diagnosis (p=0.014). Clinical response (complete and partial) was more prevalent in patients having cN0 and cN1 stage, 48.8% (n=15) each, whereas in cN2 the clinical response was seen only in one patient. Additionally, clinical stage of disease at presentation was the only variable significantly related to pathologic complete response (P=0.025). The majority of patients, 80% (n=24), that didn’t show complete pathological response had clinical stage IIIb disease, and the rest 20% (n=6) had clinical stage IIIa disease at presentation. Considering the age of patients, among those who showed complete clinical response, 22 (71%) were aged below 50 years and 9 (29%) were aged 50 years and above. However, there were no statistically significant differences in clinical and pathological response in terms of age.
Table 5: Distribution of Clinical and Pathologic Response
| Variable | Clinical response, n=41 | Pathological complete response (n=37), n (%) | ||||
|---|---|---|---|---|---|---|
| Yes | No | p-value | Yes | No | p-value | |
| Age | 22 (71%) | 9 (90%) | 0.40 | 4 (57.1%) | 23 (76.7%) | 0.36 |
| >=50 | 9 (29%) | 1 (10%) | 3 (42.9%) | 7 (23.3%) | ||
| cT staging | 1 (3.2%) | - | 0.48 | 3 (42.9%) | 1 (3.3%) | 0.07 |
| T2 | 5 (16.1%) | - | 2 (28.6%) | 14 (46.7%) | ||
| T3 | 25 (80.6%) | 10 (100%) | 4 (57.1%) | 27 (90%) | ||
| cN staging | 15 (48.4%) | 3 (30%) | 0.014 | 2 (28.6%) | 2 (6.7%) | 0.45 |
| N1 | 15 (48.4%) | 6 (60%) | 5 (71.4%) | 13 (43.3%) | ||
| N2 | 1 (3.2%) | 1 (10%) | - | 3 (10%) | ||
| Histologic sub-type | 21 (87.5%) | 5 (83.3%) | 0.61 | - | - | |
| IDC | 2 (8.3%) | 1 (16.7%) | - | - | ||
| ILC | 1 (4.2%) | - | - | - | ||
| Grade (Differentiation) | 7 (29.2%) | 3 (50%) | - | - | ||
| 2 | 7 (29.2%) | 1 (16.7%) | - | - | ||
| 3 | 10 (41.7%) | 2 (33.3%) | 2 (28.6%) | 6 (20%) | ||
| cGroup stage | 8 (25.8%) | - | 0.12 | 2 (28.6%) | 6 (20%) | 0.025 |
| IIIa | 21 (67.7%) | 10 (100%) | 3 (42.9%) | 24 (80%) | ||
age, histologic sub-type, and pathologic grade (differentiation) of tumor (p>0.05) (Table 5). Comparison between clinical and pathologic response was also done. Based on end-cycle clinical assessment, 7 of the 9 patients (77.8%) who were considered as complete clinical responders, in fact, had pathological complete response (pCR).
Discussion
In our study, the majority of patients with LABC were younger female patients, with a median age of 41 years, which is 21 years younger than the western population median (62 years). Our finding is in line with another study done in Egypt, which shows the first presentation of LABC 10 years younger than the western population with a median age of 65 years [27]. In our study, clinical stages IIIA and IIIB accounted for 8 (19.5%) and 31 (75.6%) of LABC cases, respectively. This is in concordance with previous studies done in Sudan, where the number of patients with clinical stage IIIA was 13 (13.3%) and IIIB was 77 (78.6%) [30, 14, 31, 32]. The use of NACT to treat locally advanced breast cancer has been shown to be effective. In our study, the overall clinical response rate was 75.6% (n=31). This finding was comparable to prior studies done in Sudan, Bangladesh, India, and the USA, which showed clinical response rates of 83.6%, 88%, 80.4%, and 80%, respectively [9, 24, 25, 26].
Considering nodal status, the cN stage of patients at presentation was found to be significantly associated with the clinical response (p=0.014). Clinical response (complete and partial) was more prevalent in patients having cN0 and cN1 stage—48.8% (n=15) each—whereas in cN2 the clinical response was seen only in one patient. This was in line with a study done in Bangladesh which showed that clinical response was higher in patients having cN0 and cN1 stages—100% and 91%, respectively—whereas in cN3 the clinical response was seen in only 5% of patients [24].
Considering the pathological grade (differentiation) of the tumor, in our study, 41.7% of patients who had a clinical response were Grade 3 tumors, which aligns with other studies where better responses were observed in rapidly proliferating, higher-grade tumors. Also, in our study, the majority of patients with clinical response had high-grade tumors; however, this was statistically not significant [9, 24, 28, 29, 23].
The pathological complete response (pCR) of LABC after using NACT was seen in 7 patients, which was 18.9% of those who underwent surgical intervention. Our result was comparable to prior studies done in Brazil and Egypt, which reported pCR rates of 16.5% and 16%, respectively [33, 8]. Additionally, clinical stage of disease at presentation was the only variable significantly related to pathologic complete response (P=0.025). The majority of patients—80% (n=24)—who didn’t show complete pathological response had clinical stage IIIB disease, and the rest 20% (n=6) had clinical stage IIIA disease at presentation [30, 14, 31, 32].
Considering hormonal status and cPR, prior studies show that patients with triple-negative and HER2-positive tumors have higher pCR rates. A study in the USA showed that rates of pCR in both the breast and axilla were 38.2% in triple-negative, 45.4% in HER2-positive, and 11.4% in hormone-receptor-positive, HER2-negative disease (P<0.0001). However, in our study, hormonal status was determined for only seven patients, and none of the patients with pCR had their hormonal status tested [10].
Strengths and Limitations of the Study
Strengths
- This is the first study to describe pathological complete response and clinical responses in locally advanced breast cancer patients receiving NAC.
- The study was conducted at TASH, the preferred referral hospital in the country, where patients came from all parts of Ethiopia, possibly representing the majority of the national population.
- All patients within the study period were included to increase the sample size, thereby improving the representativeness of the results.
Limitations
- Although a longer study period was chosen, the small number of patients decreased the statistical power of the results.
- Hormonal status was not determined for most study participants, making it impossible to assess the association between response and hormonal status.
Conclusions and Recommendation
Neoadjuvant chemotherapy can achieve a high clinical response, and there is also complete pathological response in some patients with locally advanced breast cancer. Although the entire patients in our study undergone MRM, it was possible to do Breast Conserving Surgery (BCS) in some of the patients after NACT. We recommend further research to find predictors for response. All patients should have core needle biopsies with hormone status determination before starting NACT, because for those patients with cPR it is impossible to do after surgery. This not only affects our prediction of response to NACT but also future adjuvant hormonal treatment of the patient. Further study to determine the contribution of response to NACT to survival should be done.


